[ad_1]
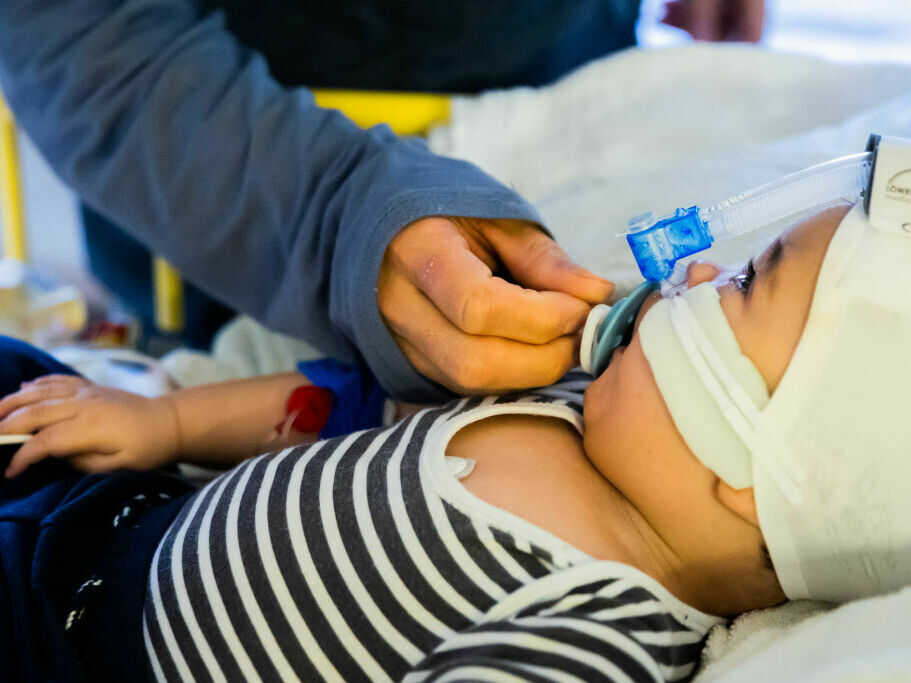
Every year, RSV infections ship as much as 80,000 youngsters underneath 5 to the hospital for emergency remedy. A brand new antibody remedy might defend the youngest youngsters — newborns and up infants as much as 2 years previous.
Christoph Soeder/dpa/image alliance through Getty I
disguise caption
toggle caption
Christoph Soeder/dpa/image alliance through Getty I
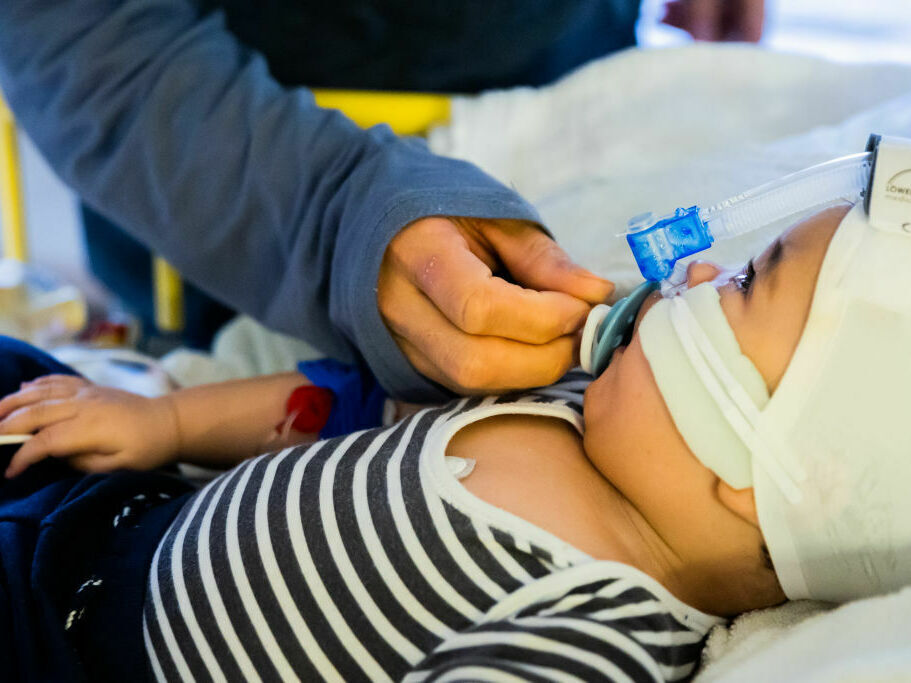
Every year, RSV infections ship as much as 80,000 youngsters underneath 5 to the hospital for emergency remedy. A brand new antibody remedy might defend the youngest youngsters — newborns and up infants as much as 2 years previous.
Christoph Soeder/dpa/image alliance through Getty I
Cheryl Meany, a highschool instructor from Camillus, N.Y., was excited when she realized she was carrying twins in 2014. However her pleasure shortly turned to fret as medical doctors flagged a number of well being considerations, together with attainable mind lesions.
So she wanted a second to course of when her husband, a respiratory therapist, proposed enrolling the soon-to-be-born infants in an experimental examine for an unrelated sickness. It was a trial for a protecting remedy for RSV or respiratory syncytial virus, a standard respiratory virus that may be fairly extreme in younger youngsters.
“It took me aback, like ‘What are you even speaking about? I do not even know what you are asking me proper now,'” Meany stated.
That was in 2014, a number of years earlier than the latest RSV surge overwhelmed hospitals throughout the nation. However Meany was nervous concerning the sickness again then after seeing a few of her associates’ youngsters find yourself within the hospital from it. As much as 80,000 youngsters underneath 5 are admitted for RSV annually.
So she enrolled her daughters within the trial for a monoclonal antibody that works to forestall RSV-induced decrease respiratory tract an infection in infants. Her choice helped transfer ahead probably the most promising therapies to guard infants from RSV in many years.
In January, drugmakers AstraZeneca and Sanofi introduced the U.S. Meals and Drug Administration is formally reviewing their utility to get the remedy – known as nirsevimab – authorised within the U.S., together with outcomes from the trial the Meany twins joined.
AstraZeneca stated its third section trial outcomes confirmed its single-dose remedy was practically 75% efficient at stopping extreme an infection in infants all through an RSV season. The info was revealed in March 2022 within the New England Journal of Drugs.

Earlier than they have been born in 2015, twins, Stella and Cassidy Meany (left to proper), have been enrolled in a trial for a preventative remedy for RSV. The remedy might quickly be obtainable to guard newborns towards the respiratory sickness.
Cheryl Meany
disguise caption
toggle caption
Cheryl Meany

Earlier than they have been born in 2015, twins, Stella and Cassidy Meany (left to proper), have been enrolled in a trial for a preventative remedy for RSV. The remedy might quickly be obtainable to guard newborns towards the respiratory sickness.
Cheryl Meany
Dr. William Schaffner, medical director on the Nationwide Basis for Infectious Ailments who was not concerned on this analysis, stated the outcomes counsel nirsevimab might considerably cut back the numbers of infants which can be hospitalized annually for RSV.
“The potential affect in assuring a wholesome infancy for a really giant proportion of the infants born right here in the USA — and even past — is probably very, very giant,” Schaffner stated.
The drug – a long-lasting antibody injection – is meant for newborns or different infants going through their first RSV season, and for infants as much as 24 months of age of their second RSV season, in line with AstraZeneca’s press launch.
Dr. Joseph Domachowske, a pediatric infectious illness specialist at Upstate Medical College Hospital in Syracuse, helped launch the earliest section of the nirsevimab examine.
“RSV is the primary cause why infants and younger youngsters are hospitalized, not simply within the U.S., however the world over,” he stated.
Domachowske, who additionally led the hospital’s COVID-19 vaccine trial for youths, expects a greenlight from regulators in time to have nirsevimab obtainable by the subsequent RSV season within the fall. It has already been authorised in Europe.
A protracted journey to develop an efficient remedy
When Meany’s daughters acquired their injections in January 2015, they have been the primary infants on the planet to obtain it, in line with AstraZeneca.
Domachowske, a Meany household pal, stated giving the dual infants safety towards RSV was a big second after researchers had struggled for years to discover a remedy to forestall RSV. Again within the Sixties, a completely different remedy, a vaccine candidate, was underneath examine. Nevertheless it made youngsters sicker from RSV – and two infants died from it.
“It actually charged up the mistaken half of the immune system,” Domachowske stated.
Progress did not come till 20 years later. In 1998, the FDA OK’d a monoclonal antibody for untimely and high-risk infants. However Domachowske stated altering medical tips since then have severely restricted eligibility for this remedy, and, he stated, its efficacy wasn’t nice.
“It needs to be given month-to-month,” Domachowske stated. “And it is efficient at stopping hospitalization, not efficient at stopping an infection.”
That is the place the analysis had been caught for years till 2014, when Domachowske attended a medical convention in Argentina. A featured speaker dropped a large discovery that loads of RSV analysis centered on the mistaken protein.
“Everyone seems to be sitting there staring with their mouths gaping open like, ‘That is why all of our work hasn’t led to something for many years,” Domachowske stated. “It was that spectacular. And you’ll see the pharma people who have been attending, taking notes, calling their colleagues saying, ‘Cease, cease the work.'”
Not too lengthy later, he injected Meany’s daughters with an improved, longer-lasting monoclonal antibody that protects infants by means of an RSV season with one shot.

The Meany twins have been the primary on the planet to get pictures of nirsevimab throughout early trials after they have been infants. They’d no unwanted effects and no signs of RSV.
Cheryl Meany
disguise caption
toggle caption
Cheryl Meany

The Meany twins have been the primary on the planet to get pictures of nirsevimab throughout early trials after they have been infants. They’d no unwanted effects and no signs of RSV.
Cheryl Meany
The dual ladies, Cassidy and Stella, at the moment are 8 years previous and wish to compete in ninja warrior contests — they race by means of impediment programs that characteristic ladders, monkey bars and overturned Bosu balls.
Meany stated the women by no means had issues from the shot and by no means displayed signs of RSV. She is pleased with the function they performed in medical historical past.
“This issues, and this issues for youths in all places, not simply youngsters right here,'” Meany stated.
Domachowske stated the women might have gotten RSV in later seasons after the results of the remedy had worn off. However since older youngsters’s immune methods are stronger, signs weren’t noticeable.
A welcome RSV prevention instrument
Physicians and infectious illness specialists welcome the potential approval of the remedy.
Schaffner of the Nationwide Basis for Infectious Ailments stated if it have been already authorised within the U.S., nirsevimab would’ve helped curb the excessive fee of infections seen this season, one of many worst latest seasons for the illness.
“This latest surge would have been remarkably blunted,” he stated.
Dr. Vandana Madhavan, scientific director of pediatric infectious illness at Mass Basic for Kids stated the monoclonal antibody is a big achievement within the struggle towards RSV.
“This can be a large step ahead,” she stated.
[ad_2]

