[ad_1]
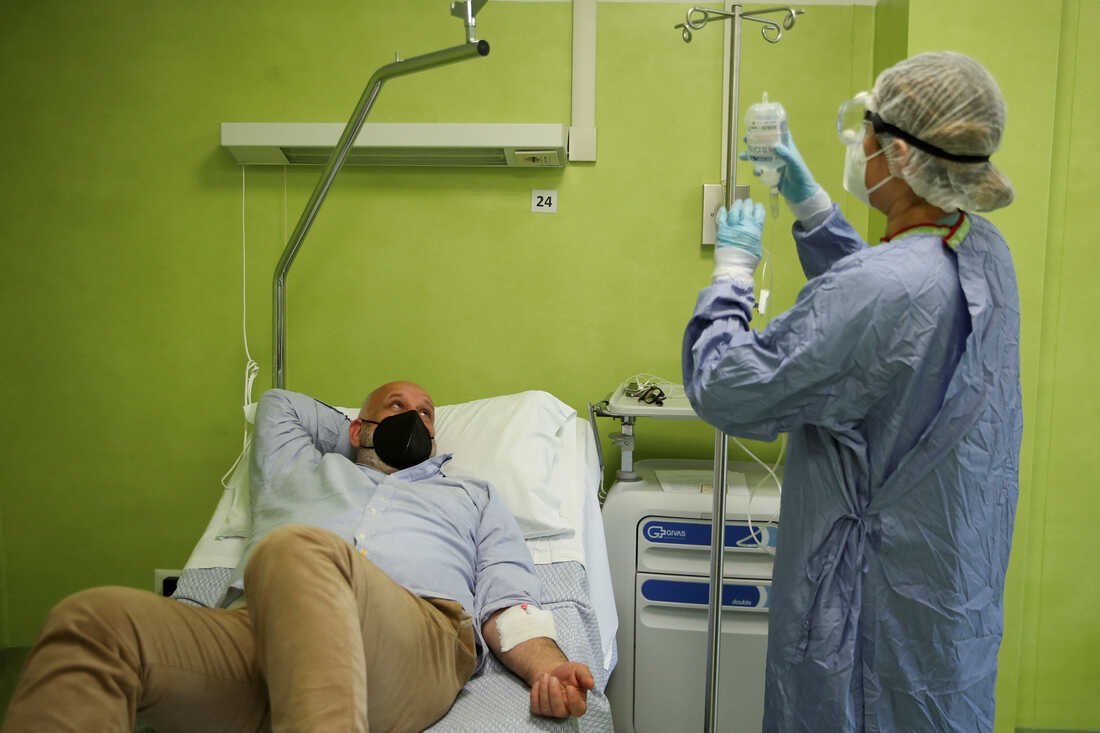
Antonio Rapuano acquired an infusion of a monoclonal antibody to deal with his COVID in Albano, Italy in 2021. Such infusions have been efficient therapies for COVID through the pandemic, however medical doctors at the moment are discovering that the majority monoclonal antibodies now not work in opposition to new variants of SARS-CoV-2.
Yara Nardi/Reuters
disguise caption
toggle caption
Yara Nardi/Reuters
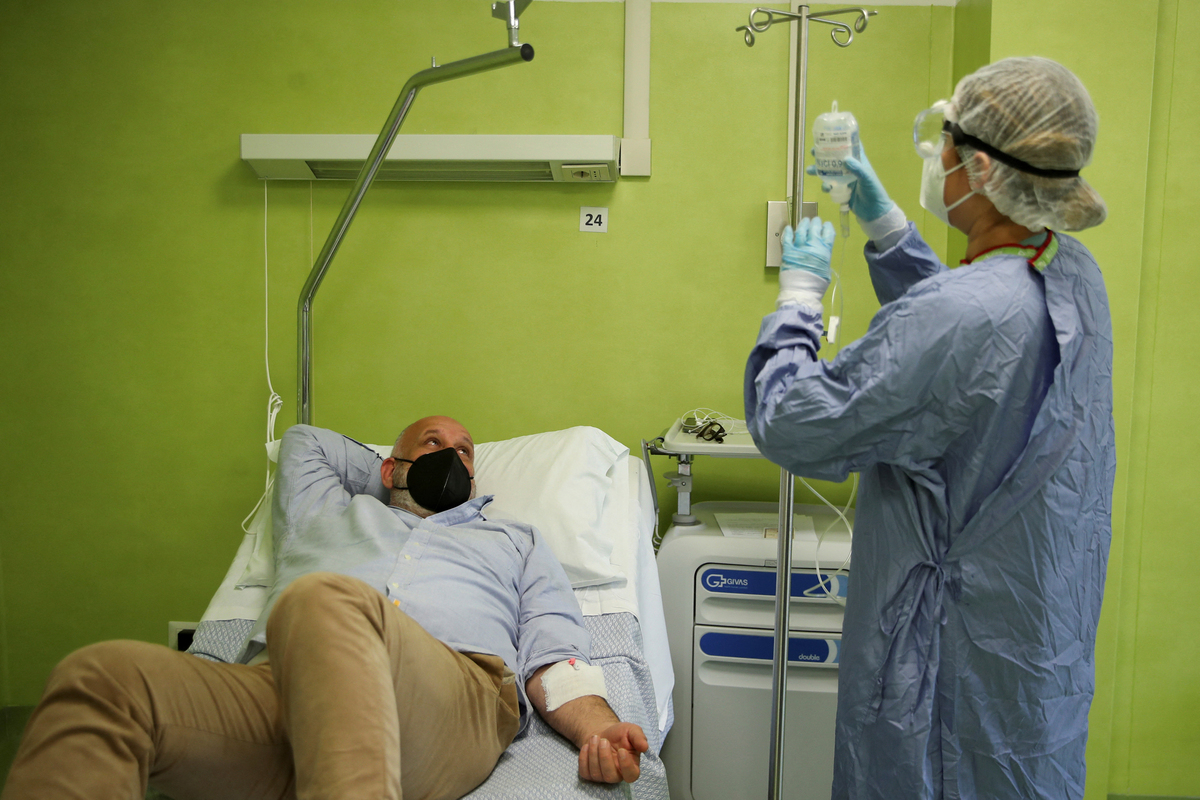
Antonio Rapuano acquired an infusion of a monoclonal antibody to deal with his COVID in Albano, Italy in 2021. Such infusions have been efficient therapies for COVID through the pandemic, however medical doctors at the moment are discovering that the majority monoclonal antibodies now not work in opposition to new variants of SARS-CoV-2.
Yara Nardi/Reuters
Monoclonal antibodies had been as soon as the star of COVID-19 outpatient therapies. Since they first grew to become out there in 2020 – even earlier than the primary vaccines – greater than 3.5 million infusions of the factory-grown proteins have been given to sufferers within the U.S. to assist scale back danger of hospitalization.
However one after the other, totally different monoclonal therapies have misplaced their efficacy in opposition to new variants of the coronavirus. The rise of Paxlovid antiviral capsules earlier this 12 months, additional dented their enchantment.
Now, a brand new wave of omicron subvariants which can be the greatest but at evading the immune system’s present defenses have taken over within the U.S. They’re anticipated to knock out bebtelovimab, the final monoclonal antibody therapy standing in opposition to the coronavirus. Quickly, it’s going to be a part of bamlanivimab, casirivimab, sotrovimab and others within the graveyard of monoclonals that after focused previous COVID strains till they had been outflanked by variants that evaded their safety.
“Monoclonals had their day, just like the Mannequin T or the biplane,” says Carl Dieffenbach, director of the Division of AIDS on the Nationwide Institutes of Well being, and lead of the NIH’s Antiviral Program for Pandemics, “Now it is time to transfer on.”
Not everybody fully agrees. Monoclonals are nonetheless helpful, some medical doctors say, for treating a weak inhabitants.
“There are severely immunosuppressed sufferers that aren’t more likely to mount an immune response to the virus, even in case you deal with them with antiviral medication,” says Dr. Raymund Razonable, an infectious illness specialist within the transplant division on the Mayo Clinic. “That is the group that’s going to be probably the most affected by the absence of antibody-based therapies.”
What’s extra new analysis is underway to develop new sorts of monoclonal antibodies that might even maintain up in opposition to new variants.
How monoclonals work — and what they’re up in opposition to
Monoclonal antibody therapies have all the time had a serious weak point – they’re simply outmaneuvered by new COVID strains. It is a flaw that is baked into how they work.
Monoclonal antibodies are lab-grown proteins that complement your physique’s immune system – which, in most individuals, is of course producing antibodies to hunt for potential threats on a regular basis.
“You and I and each human being that has a functioning immune system is strolling round with most likely trillions of completely totally different antibody molecules simply circulating in our blood,” says Derek Lowe, a chemist and blogger for the journal Science, “Each one among us has a very totally different suite of them. There are extra of them than there are stars within the sky.”
The tiny, Y-shaped proteins lurk within the blood in low concentrations, “ready and ready till they occur to stumble upon one thing that they stick to actually nicely, they usually discover their soulmate, mainly,” Lowe explains. That “soulmate” is an antigen – a international substance that is entered the bloodstream, like a bacterial protein or a virus or a pollen grain.
As soon as a monoclonal antibody finds its soulmate — within the case of COVID, a particular half on the tip of the SARS-CoV-2 virus – it binds to the floor of the antigen. Then, it sends out alerts to the immune system, “like hey, I’ve acquired a reside one,” Lowe says.
Essentially the most highly effective antibodies can cease the virus in its tracks simply by binding to it. As an illustration, “in case you have an antibody that sticks to the tip of the spike protein on the enterprise finish of the virus – simply the truth that it’s caught tightly to which means the virus can not infect a cell,” says Lowe.
The spike protein has been the goal of all of the monoclonal antibody therapies that go after the virus to date. Nevertheless it’s been a fickle soulmate, altering with new variants, leaving the monoclonal antibodies adrift within the bloodstream with nowhere to bind.
Firms have stopped bringing these monoclonals to market. The federal authorities stopped promising to purchase them in amount, making it a riskier guess for corporations.
“There are antibodies on the market, however no person has the $200 million to develop it,” Dieffenbach says, citing prices that embrace producing the antibodies, working trials and getting them approved by the Meals and Drug Administration. Some corporations figured it wasn’t value it, for a product that was more likely to change into out of date in a matter of months, he says.
To be clear, these are antibody therapies for outpatient therapy. There’s a totally different type of monoclonal antibody therapy for hospitalized sufferers that is still viable. Actemra, because it’s known as, isn’t inclined to virus mutation as a result of it targets the physique’s immune response to the virus, somewhat than the virus itself.
New instructions in analysis, and a possible comeback
There would possibly nonetheless be hope for monoclonals. Drugmakers and researchers at authorities companies at the moment are retooling the technique, on the lookout for monoclonal antibodies that might final.
“Initially, the main focus was, ‘let’s simply discover probably the most potent antibodies,'” says Joshua Tan, chief of the Antibody Biology Unit at NIH. “Now, there’s consciousness that we have to discover antibodies which can be more likely to work in opposition to not simply the [current version of the] coronavirus, however no matter could come.”

Joshua Tan, Chief of the Antibody Biology Unit at NIH.
Pien Huang/NPR
disguise caption
toggle caption
Pien Huang/NPR
In his lab in Rockville, Md., Tan and the researchers who work with him are on the lookout for antibodies that concentrate on components of the virus which have stayed the identical on a number of totally different viruses inside the bigger coronavirus household. “We’re different components of the spike protein which may be extra constant and could also be tougher to mutate,” Tan says.
To realize this, researchers in Tan’s lab are taking immune cells from the blood of sufferers which have recovered from COVID, and pelting them with tiny plastic pellets lined with spike proteins from totally different, older coronaviruses to see which cells reply. “Not the [COVID] variants, however SARS-CoV-1, SARS-CoV-2, MERS [etc.],” post-doctoral researcher Cherrelle Dacon clarifies. “These are seven totally different coronaviruses, all of which infect people.”
The immune cells that react to a number of totally different coronaviruses are making antibodies that bind to part of the spike protein that is staying the identical throughout them.
It is a painstaking course of: Isolating particular person immune cells, discovering those that make antibodies in response to varied spike proteins — after which utilizing these to make extra antibodies that they will scale up, analyze and take a look at, to determine what on the virus they’re truly binding to. The method takes about three to 4 months every cycle, Tan says.
Tan says the excellent news is that they’ve discovered some antibodies that keep on with a number of totally different coronaviruses. They printed a number of the outcomes earlier this summer season in Science.

Proper: Tan holds a chip able to be loaded with immune cells that will probably be be sorted and examined in opposition to totally different viruses. Left: The display of the Beacon, a machine that isolates particular person immune cells so researchers can take a look at which of them reply strongly to a couple of coronavirus.
Pien Huang/NPR
disguise caption
toggle caption
Pien Huang/NPR
However the issue the researchers have come up in opposition to is that the monoclonal antibodies they’ve discovered should not so potent. Tan says there appears to be a tradeoff – between how nicely a monoclonal antibody in opposition to COVID-19 works, and the way lengthy it lasts earlier than the virus ditches the antibody’s goal.
An analogy: If the coronavirus had human physique components (which it would not) the previous, extremely efficient monoclonals hit the virus’s spike protein squarely on the nostril. In distinction, the brand new monoclonals Tan is discovering attempt to seize it by the armpit. “One of many points seems to be that it is tougher to achieve these components,” Tan says, “What the broader, much less potent [antibodies] want is for the spike protein to shift in form” to ensure that them to seize it.
Tan is working to seek out methods round this tradeoff. He says you’ll be able to probably modify the antibody, change out components of it to extend its efficiency – a course of that is largely theoretical in the mean time, and can take a while to work out.
So whereas Tan and different researchers work on the subsequent era of monoclonal antibodies – ones that work nicely in opposition to every kind of coronaviruses, possibly even future pandemic ones – the nation is coming into a protracted lull with no monoclonal antibody therapies that work in opposition to dominant strains of SARS-CoV-2.
“The frustration is there since you’re shedding a very good drug,” says Razonable. “However you concentrate on the subsequent choices. The virus adapts, and we additionally adapt primarily based on what we’ve got out there.”
Fortunately, as Tan and others pursue the lengthy recreation with antibodies, there are different therapies, like Paxlovid capsules and remdesivir infusions, that also work in opposition to COVID.
And the analysis on and speedy improvement of antibody therapies has opened up prospects past COVID. “It has improved the manufacturing of monoclonals for most cancers, for immunologic illnesses,” says Dieffenbach, “It may be simpler to supply monoclonals sooner or later due to the teachings realized from SARS-CoV-2. Nothing was wasted right here.”
[ad_2]

